Techs reveal the workaround in a process involving a toxic chemical
Think of the last time you attended a game of curling or hockey. There you were, seated comfortably enough in the stands that you might have been drinking a cold beverage of one kind or another. Despite it being relatively warm in the stands, players glided or skated on a sheet of solid ice below.
How is this possible? Refrigeration, of course!
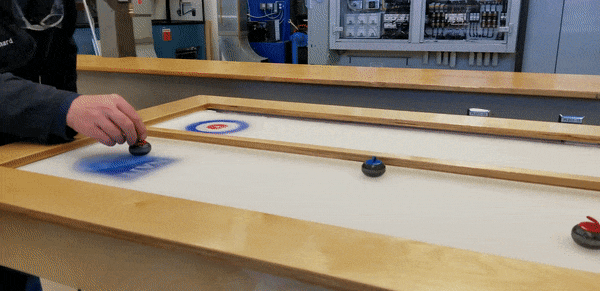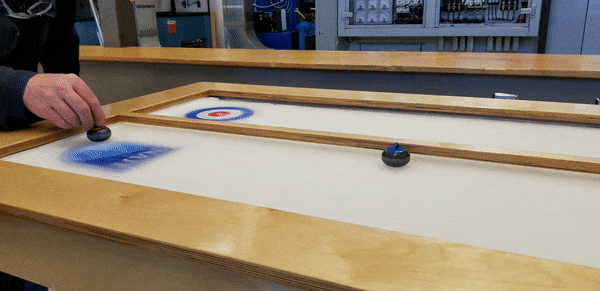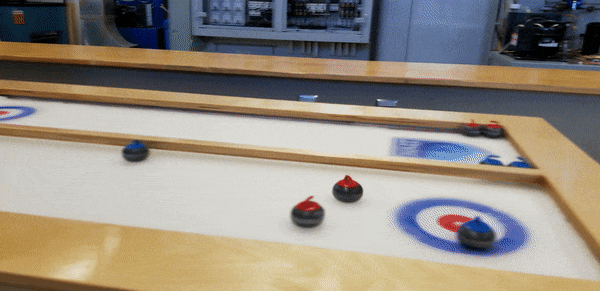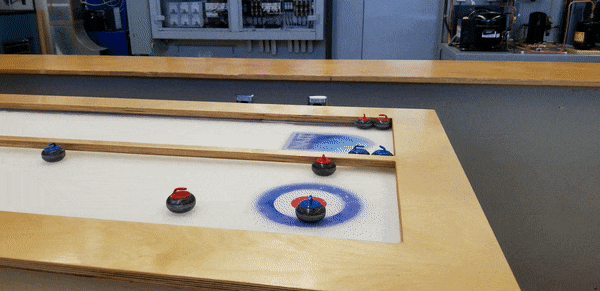
To demonstrate, staff from the HVAC, Refrigeration and Air Conditioning Mechanic and Building Environmental Systems Technology programs – including educational lab techs Duncan Albury and Richard Pelletier (HVAC ’07, BEST '08), along with instructor Justin Evernden – built a self-contained tabletop curling rink featuring a ⅜”-inch thick sheet of ice, four feet long and two wide.
After roughly two years of design and construction during their spare time, the team debuted the unit in October 2022 at NAIT’s Open House. Their hope was to introduce would-be students to the lesser known applications of the trade.
In a city where ice serves as a cultural touchstone, particularly with respect to hockey, the inner workings of a rink may surprise many. “You see an ice sheet but don’t necessarily realize what’s going on to make [it] happen inside of a warm building,” says Evernden.
“How do you do that artificially, inside a heated space?”
With their unique model as a guide, here’s their explanation.
How an indoor ice rink is made

The trick is to cool in stages. The need for the stepwise approach rests in two fascinating facts about the nitrogen-based chemical, ammonia.
Fact No. 1: Not only is ammonia perfect for cleaning windows to a streak-free shine, it’s one of the world’s most efficient refrigerants, needing less energy to facilitate rapid cooling. It’s used in heated arenas the size of Rogers Place because it can greatly lower the cost of making and maintaining ice.
Fact No. 2: The trouble is, ammonia is extremely toxic, potentially irritating and burning the skin, eyes, lungs and more. Therefore, it can't be used anywhere in the vicinity of spectators.
“If there’s an ammonia leak, you’ll take out the whole arena,” says Albury.
For that reason, as nice as it would be to simply run the ammonia through the piping in the concrete that underlies an ice surface, you can’t do it. But its power can still be harnessed.
This is where the two stages come in. Isolated in a sealed utility room, ammonia can be used as the primary refrigerant to cool a secondary one, such as a salt solution or glycol. This can then be quickly and deeply chilled to make the trip to freeze the ice without the risk of asphyxiating fans.
That’s the mechanism shown by the team’s model. Beneath their tabletop sheet of ice (the wooden cover, incidentally, was built by Carpenter program staff, while the NAIT shield and target decals were supplied by Graphic Communications), is the site of a compact refrigeration system using low-toxicity liquid R404A refrigerant.
In Stage 2, R404A, as the primary refrigerant, does the job that ammonia would do in the big leagues (where it would be referred to as R717 refrigerant), and chills, in this case, glycol. That glycol is contained in a small tank through which the R404A runs within a spiral of copper tubing. The cold glycol is then pumped out of the tank as the secondary refrigerant to a network of piping in a thin concrete slab, bringing it to a temperature of about -15 C.
This slab freezes the ice above it and the glycol recirculates to the tank to be cooled again.
“This is the principle used to build actual arena ice surfaces,” says Albury. “Basically, we’ve done the same thing.”
The team’s mini ice surface can be ready in less than eight hours, or about a quarter of the time for a real rink, which is usually twice as thick.
Sparking a conversation

Just as the system reveals the secrets of professional ice-making, it offers a rarely seen look at the field of refrigeration.
Among those who paused at Open House to slide stones toward the targets, “it sparks a conversation,” says Evernden.
Often, that involves some correction. A refrigeration mechanic might be able to repair a kitchen appliance, but that’s not what they’re trained to do.
On the job, they’re working on massive industrial chillers, such as those at warehouses or processing plants, on large-scale commercial heating, air conditioning and ventilation systems, residential air conditioning systems, or restaurants and food facilities.
Learn more about NAIT's Refrigeration and Air Conditioning Mechanic program
Or, of course, on ice rinks.
Stop the flow of refrigerant and all of those processes break down. Stop it on the team’s model, and the resulting melt might be taken for the current state of the workforce, as refrigeration veterans retire en masse. In that sense, the rink represents an overlooked opportunity.
“We’re losing people faster than they’re coming in,” Evernden says of his trade.